- Test Code: O001 (G-NIPT Lite)
- Test Code: O002 (G-NIPT Basic)
- Test Code: O003 (G-NIPT Premium)
Test Description
The non-invasive prenatal test(NIPT) is a screening test for chromosome aneuploidies from the fetal DNA present in maternal blood. Down syndrome (trisomy 21), Edward syndrome (trisomy 18), Patau syndrome (trisomy 13), sex chromosome aneuploidy, and microdeletion/microduplication are detected by using next-generation sequencing (NGS) at 10 weeks gestational age.
Ordering information
- Turnaround time: 3~5 days
- Test Timing : At 10 weeks ~ 22 weeks of pregnancy
- Specimen:
- Whole Blood 10mlStreck or Roche Tuble
⚠ Samples with Maternal cell hemolysis cannot be used for the test.
⚠ This test is for screening purpose, it is not a diagnostic test
⚠ NIPT has high sensitivity and specicity, but the possibility of false positive and false negative cannot be eliminated completely.
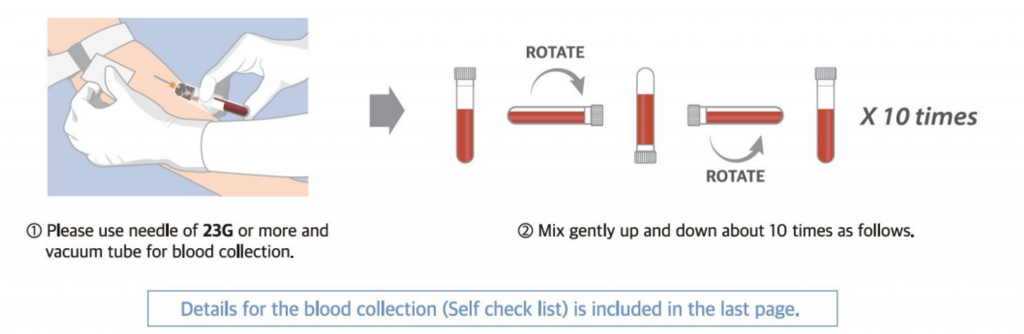
- Sample Shipping

⚠ It is recommended that the mother should take enough rest for more than 30 minutes.
⚠ When you handle the sample tube, DO NOT FREEZE
Learn more
Our own AI algorithm allows us to detect chromosomal abnormalities with a simple blood draw, providing you with peace of mind and empowering you to make informed decisions for your family’s future. we deliver fast and reliable results, coupled with compassionate support throughout your pregnancy journey
Assay information
- Test Method: Next Generation Sequencing (NGS)
- Test Subject: Fetal Trisomy (Chromosome 21, 18, 13, 9, 16, 22), Sex Choromosome Aneuploidies, Microdeletion Syndrome (>7Mb)
- Specimen Type: cfDNA tube WB 10mL
- Bioinformatics Pipeline: NIPT.v1.2
Result report

The test results will be provided according to the designated TAT. Review the sample report to see how the results are structured.
Limitations
- The purpose of this test is for risk assessment of common fetal trisomies 21, 18, 13 and sex chromosome aneuploidies. This test is performed by massivelyparallel sequencing for whole-genome using circulating cell-free fetal DNA in maternal plasma and it is possible to detect abnormalities in all chromosomes as well as chromosome 21, 18 and 13. NIPT performance is superior to the existing prenatal multiple marker screening tests.
- This test cannot identify neural tube defects and polyploidy such as trioploidy and tetraploidy.
- This test does not report monosomy. Fetal sex is not reported for twins.
- In case of trisomy 9, 16, 22 and microdeletion syndrome, the clinical sensitivity was not determined due to low incidence, and the sensitivity may be significantly affected by factors such as fetal DNA fraction and microdeletion size.
- This test is not to verify fetal karyotypes but is to determine the risk of fetal aneuploidies. If the result is positive, confirmatory test such as fetal karyotyping should be performed. Moreover, this is not a diagnostic test which does not rule out probability of false positive or false negative results.
- The factors affecting accuracy of this test are as follows: low fetal DNA fraction (early gestational weeks and high maternal BMI), undetermined maternal chromosomal abnormalities, confined placental mosaicism, fetal chromosomal mosaicism, multiple gestation, arithmetic error of calculating fetal DNA fraction, and maternal status (cancer, blood transfusion, transplantation, chemotherapy, stem cell treatment, or autoimmune disease), etc.
Verifying more specific details about the Test
